Abstract
Background The treatment of multiple myeloma (MM) continues to evolve. New therapies provide the best responses and overall survival, however, the benefit may be counterbalanced by the appearance of side effects and their severity, such as gastrointestinal toxicities, musculoskeletal pain, and central and peripheral nervous system compromise (chemo brain and neuropathy). With more data supporting the efficacy and depth of response of quadruplet (quad) therapy in patients with MM (PMID: 30694857), this study investigated the increasingly important question: does quad induction therapy increase the prevalence or severity of side effects as compared to triplet (tri) therapy treatment regimens?
Methods HealthTree® Cure Hub for Multiple Myeloma, a patient advocacy platform designed to allow patients to exchange personal experiences of disease and treatments, was used to analyze validated real-world-data concerning side effects and severity of patients receiving quad or tri therapy during induction. Patients who received fewer than tri or more than quad during induction therapy were excluded. A Pearson's Chi-squared test was performed to investigate differences between the proportions of each side-effect experienced and a two-sample t-test was used to evaluate the differences between side-effect severity (1 - hardly noticeable, 10 - unmanageable, stopped treatment).
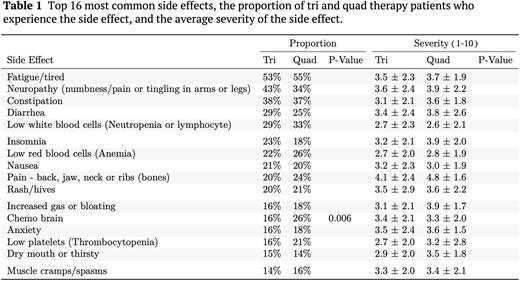
Results There were 1,204 total MM patients analyzed in this retrospective study. A majority of the patients analyzed receive tri therapy during induction (90.4%), while the remaining received quad therapy (9.6%). The three most common tri therapies in this analysis were lenalidomide, bortezomib, and dexamethasone (RVd, 69.7%); cyclophosphamide, bortezomib, and dexamethasone (CyborD, 13.2%); and carfilzomib, lenalidomide, and dexamethasone (KRd, 9.8%). The three most common quad therapies were daratumumab+RVd (28.7%), daratumumab & hyaluronidase+RVd (23.5%), and cyclophosphamide+RVd (CyRVd, 8.7%). The side effects and their severity were reported by 984 patients (82%). The analysis of side effects and severity included all tri and quad induction therapies received by patients in this study. There were 16 side effects that were reported by ≥15% of patients (Table 1). One third or more of patients reported fatigue/tired, neuropathy, constipation, diarrhea and low white blood cell count for both tri and quad therapies. The side effect of chemo brain was significantly greater (p = 0.006) in quad therapy patients (26%) than tri therapy patients (16%). Promisingly, the severity of side effects were not different between the two therapies. However, the most severe side effect reported by both therapies was musculoskeletal pain with an average score of 4.8 ± 1.6 (Quad) and 4.1 ± 2.4 (Tri).
Conclusion Surprisingly, the addition of a fourth drug to a myeloma treatment regimen did not affect the severity or, on a whole, the prevalence of the side effects experienced by patients. Importantly, 12 reported side effects were on average uncomfortable but manageable or disruptive to my life in >15% of patients. Therefore, patients’ treatment strategies, regardless of the number of drugs used, should include plans to help address treatment disruption (intensity and duration) of these common side effects, while aggressively working to achieve durable disease control.
Disclosures
Ahlstrom:Pfizer, BMS, Janssen, Takeda Oncology, Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal